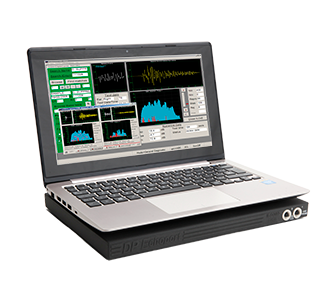
Echoport ILO292-II
Advanced binaural clinical/research OAEs
- Product No: NS-1006
- Manufacturer: Otodynamics
- OAE TEOAE DPOAE SOAE DP
- Request Quote
Description
Lightweight, portable OAE instrument for use with laptop or desktop PC via USB port. Includes both ILOV6 Clinical OAE software and EZ-Screen, with patient data management software. The 292 performs the full range of single-probe clinical OAE measurement with trusted ILO analysis.
System Features
- TEOAE, DPOAE, SOAE, DP Growth, Bilateral TE and DP plus Contralateral Suppression
- Advanced binaural clinical/research OAEs
TEOAE recording:
- Quick Screen and Standard ‘ILO’ (20ms) diagnostic recording
- Click stimulus or configurable tone pulse
- Configurable stimulus intensity. In-the-ear calibration
DPOAE recording:
- High resolution DPgram up to 8 points per octave (27 frequencies shown)
- Total DP power assessment
- DP I/O growth rate analysis, dB/dB or scissor optimized stimuli
Advanced functions:
- Powerful ‘synchronised’ spontaneous emission search to reveal actual and latent spontaneous OAEs
- Full spectrum display during DP test shows higher order DPs
- Contralateral noise suppression of TEOAE
Technical Specifications
|
Dimensions: |
297mm x 210mm x 18mm (11.75in x 8.25in x 0.75in) |
|
Weight: |
1.045kg (2.3lb). Connections: Two probe sockets - for UGS or UGD probes with built in ID and memory chip. (292 USB-I one probe socket fitted. Removable blank for upgrade). Standard USB interface on rear. |
|
Power consumption: |
50mA when device connected; 140mA when software running; 320mA recording monaural; 480mA recording binaural |
|
Channels: |
4 Independent stimulus channels (292-I two only) 16 bit 25kHz, 0-95dB. 2 signal input channels (292-I one only) 16 bit 25kHz |
|
PC requirements: |
Windows 10 and 7, free USB port |
|
Classification: |
Type BF, class II. Certified as meeting EN60601-1, UL2601, CSA 22.2, no. 601-1, CE, EMC. Otodynamics is an ISO9001 and ISO13485 compliant company. |